solution of aldehyde ketone and carboxylic acid Carboxylic acids acid aldehydes ketones structure compounds derivatives aldehyde ketone ester reduction structures synthesis britannica amide anhydride acyl chloride reagents
Hey there! Today I want to talk to you about two interesting topics in the field of chemistry. The first one is all about classifying different substances as alcohols. The second topic focuses on aldehydes, ketones, and carboxylic acids. Let’s dive into these concepts and explore them in detail!
Alcohols - A Closer Look
 Alcohols are organic compounds that contain a hydroxyl (-OH) group bonded to a carbon atom. They are an essential part of our daily lives, as many alcoholic beverages such as beer, wine, and spirits contain them. However, alcohols are not limited to just drinks; they also play a crucial role in various industrial processes.
Alcohols are organic compounds that contain a hydroxyl (-OH) group bonded to a carbon atom. They are an essential part of our daily lives, as many alcoholic beverages such as beer, wine, and spirits contain them. However, alcohols are not limited to just drinks; they also play a crucial role in various industrial processes.
Let’s take a closer look at how we can classify different substances as alcohols. To determine if a compound is an alcohol, we need to check for the presence of the hydroxyl group attached to a saturated carbon atom. If this group is absent, the compound is not an alcohol.
Alcohols can be classified into different categories based on the number of hydroxyl groups present. Monohydric alcohols, as the name suggests, contain one hydroxyl group per molecule. Ethanol, commonly known as drinking alcohol, is a prime example of a monohydric alcohol.
On the other hand, dihydric alcohols, such as ethylene glycol, contain two hydroxyl groups per molecule. These compounds find extensive use as coolants and antifreeze agents due to their excellent heat-transfer properties.
Finally, there are polyhydric alcohols like glycerol, which contain three or more hydroxyl groups. Glycerol has various applications in the pharmaceutical and cosmetic industries because of its moisturizing properties.
Aldehydes, Ketones, and Carboxylic Acids
 Now let’s move on to the fascinating world of aldehydes, ketones, and carboxylic acids, which are part of the functional group known as carbonyl compounds.
Now let’s move on to the fascinating world of aldehydes, ketones, and carboxylic acids, which are part of the functional group known as carbonyl compounds.
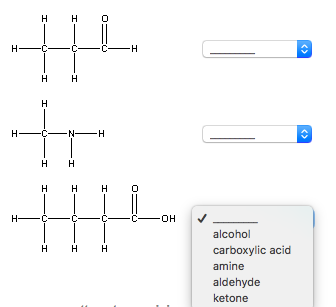
Aldehydes and ketones both contain the carbonyl group (C=O), but they differ in their placement within the molecule. In aldehydes, the carbonyl group is located at the end of the carbon chain, whereas in ketones, it is found in the middle. Carboxylic acids, on the other hand, have a carboxyl group (-COOH) attached to a carbon atom.
These compounds have various applications in different fields. Aldehydes, such as formaldehyde, have widespread use as preservatives, disinfectants, and even in the production of plastics. Ketones, like acetone, are famous for their solvent properties and find applications in paints, varnishes, and nail polish removers.
Carboxylic acids, such as acetic acid (vinegar), are commonly used in food preservatives, pharmaceuticals, and the manufacturing of polymers. They also play a vital role in biological processes, as they are involved in the metabolism of fats and carbohydrates.
Understanding the properties and characteristics of alcohols and carbonyl compounds broadens our knowledge of the diverse world of chemistry. By classifying substances and studying their structures, we can unlock their potential applications in various industries.
That’s it for today’s discussion! I hope you found this brief overview of alcohols, aldehydes, ketones, and carboxylic acids insightful. Chemistry is a truly fascinating subject, full of endless possibilities and discoveries. Keep exploring and stay curious!
If you are looking for 5D.7 Aldehydes and Ketones (OC) – CdV MCAT Review you’ve came to the right web. We have 5 Pics about 5D.7 Aldehydes and Ketones (OC) – CdV MCAT Review like carboxylic acid - Reduction | Britannica, 5D.7 Aldehydes and Ketones (OC) – CdV MCAT Review and also carboxylic acid - Reduction | Britannica. Read more:
5D.7 Aldehydes And Ketones (OC) – CdV MCAT Review
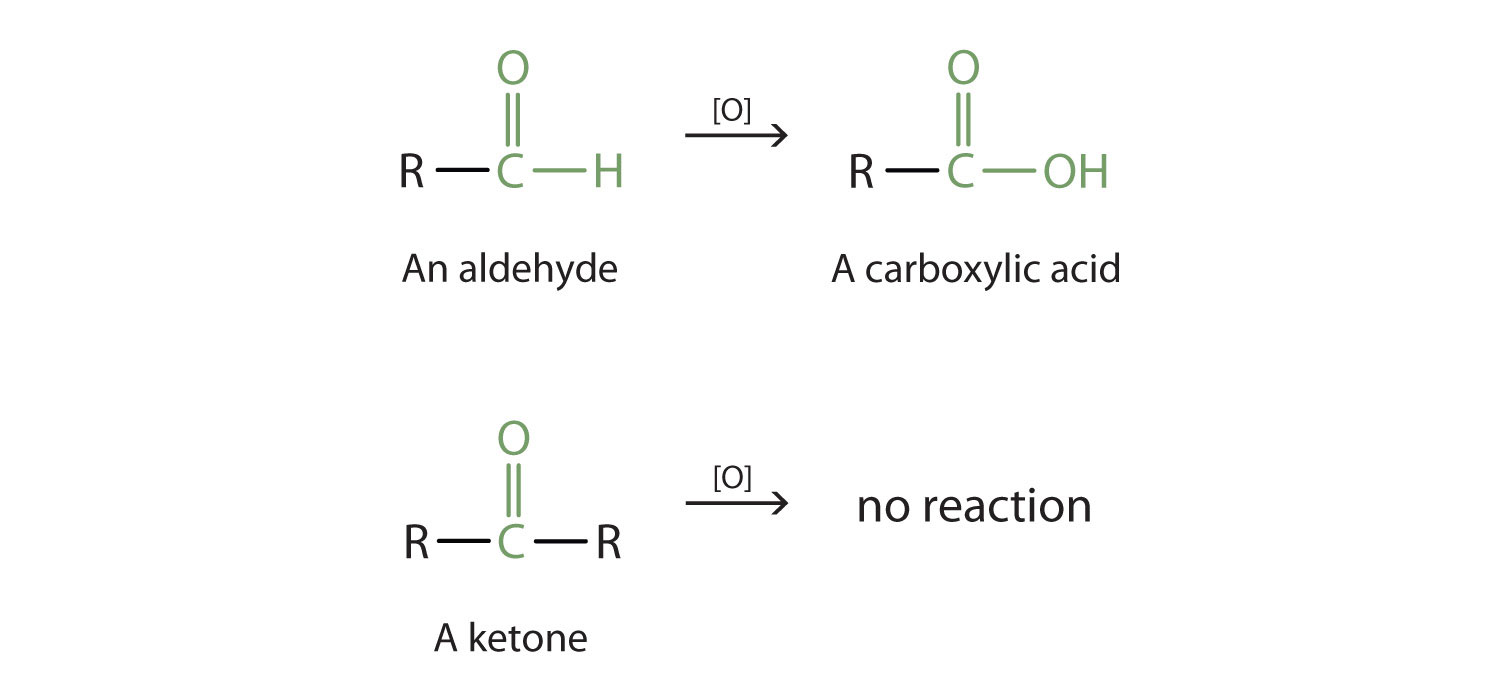 cdvmcat.wordpress.comaldehydes oxidation organic ketones oxygen acid carboxylic compounds reaction oxidized acids chemistry general most properties ketone oxidizing group carbonyl reactions
cdvmcat.wordpress.comaldehydes oxidation organic ketones oxygen acid carboxylic compounds reaction oxidized acids chemistry general most properties ketone oxidizing group carbonyl reactions
Carboxylic Acid - Reduction | Britannica
 www.britannica.comcarboxylic acids acid aldehydes ketones structure compounds derivatives aldehyde ketone ester reduction structures synthesis britannica amide anhydride acyl chloride reagents
www.britannica.comcarboxylic acids acid aldehydes ketones structure compounds derivatives aldehyde ketone ester reduction structures synthesis britannica amide anhydride acyl chloride reagents
Solved Classify Each Of The Following As An Alcohol, A | Chegg.com
 www.chegg.comaldehyde amine classify ketone carboxylic
www.chegg.comaldehyde amine classify ketone carboxylic
12th CLASS CHEMISTRY CHAPTER-12 ALDEHYDE KETONE AND CARBOXYLIC ACID
 www.youtube.comketone aldehyde carboxylic
www.youtube.comketone aldehyde carboxylic
Solved Classify Each Of The Following As An Alcohol, A | Chegg.com
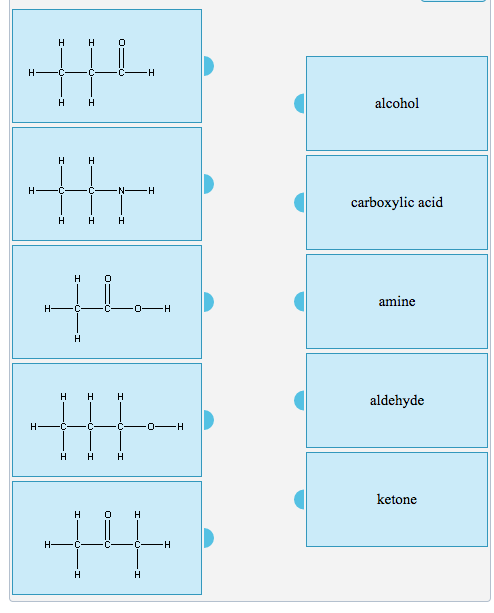 www.chegg.comketone carboxylic aldehyde amine transcribed
www.chegg.comketone carboxylic aldehyde amine transcribed
Ketone aldehyde carboxylic. Ketone carboxylic aldehyde amine transcribed. Carboxylic acid